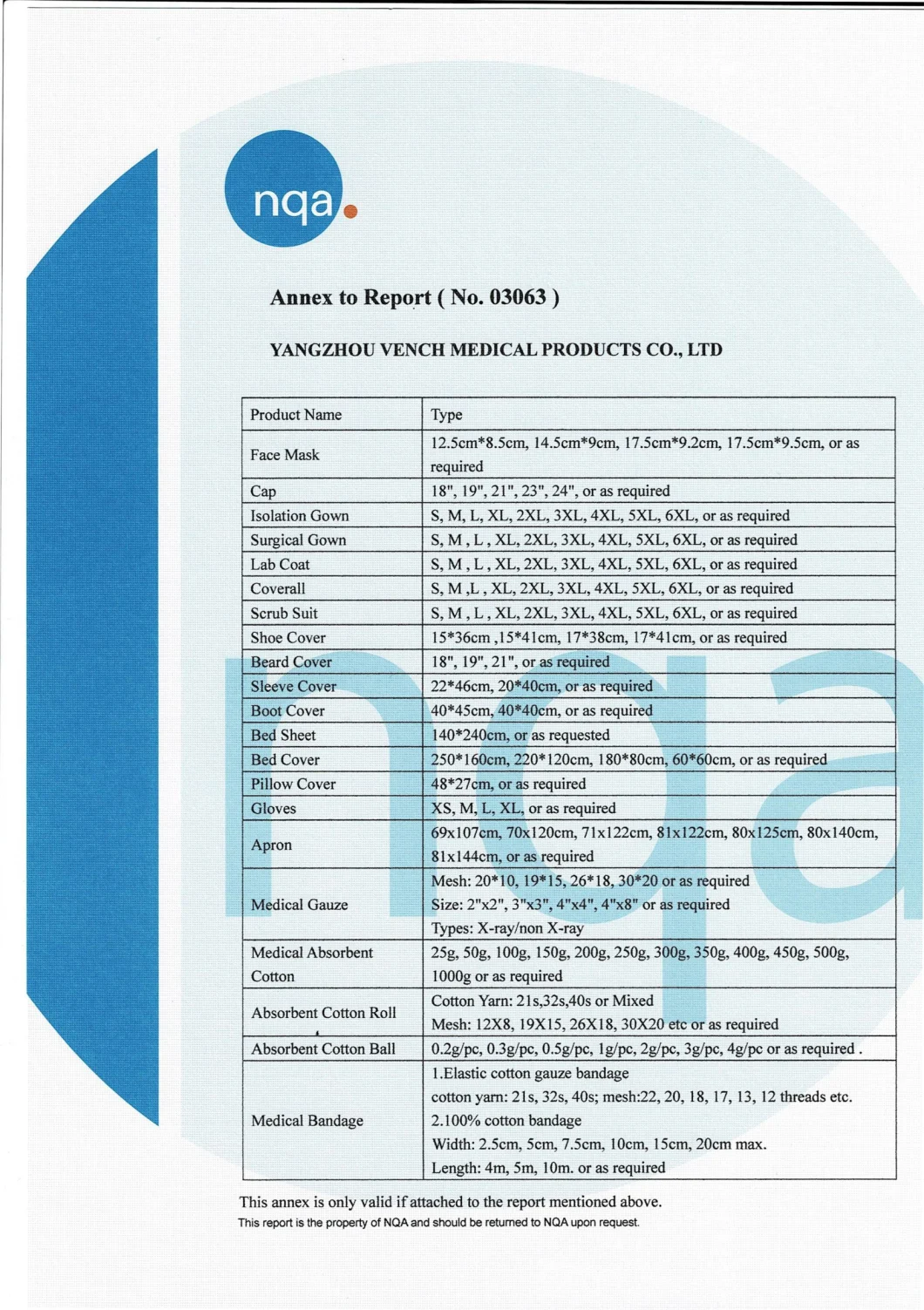
| NO. | PRODUCT NAME | SIZE(CM) | UNIT | |||
| 1 | Table drape with adhesive tape | 50*50 | 1 | |||
| 2 | Instrument table cover | 80*80 | 1 | |||
| 3 | Dental patient gown(with velcro) | 65*110 | 1 | |||
| 4 | Reflector drape | 15*15 | 2 | |||
| 5 | Transparent hose cover | 10*120 | 2 | |||
| 6 | Gauze swabs | 7.5*7.5 | 10 | |||
| 7 | Standard Ult.Stitch Surgical Gown | 130*150 | 1 | |||
| 8 | Bundle cover | 100*100 | 1 | |||
| Specification of disposable sterile implant pack | ||||||
| Model NO. | VDSIP | |||||
| Material | SPP, SMS | |||||
| Trademark | VMED | |||||
| Main usage | wound management | |||||
| Certification | CE, ISO, FDA | |||||
| Loading Port | Shanghai | |||||
| Package | 1set/blister bag EO STERILE 30bags/ctn | |||||
| Carton size(cm) | 60*40*45 | |||||
| Weight(kg) | 10.5 | |||||
| HS code | 3005901000 | |||||
| Logo | OEM | |||||
| Delivery | within 30days | |||||
| Quality Guarantee | 5 years | |||||
| Origin | China | |||||

Certifications




FAQ
Q1: Can I have a sample first ?
VMED:Of course ! Sample are available and free
Q2: Do your company have any quality management system such as ISO, EN or CE certificate(s)?
VMED: Yes, we have CE, ISO13485, FDA,
Q3:What's your advantage? Why we choose you?
VMED: Vench Medical Products Co., Ltd. is a fast growing company, specializing in the manufacture of disposable medical products and personal protection products.
Our company has implemented a strict quality management system (QMS) and has passed ISO13485 certification. Our main products have passed CE certification of European Union (EU) and FDA registration of USA.
Q4 : How does your factory control the quality?
VMED : All manufacturing are absolutely base on the pre-production samples. Meanwhile, we have a complete after-sales system.
Q5: What the expiration date for disposable non-woven products?
VMED: We suggest 2 years of shelf storage
Trust us
-- professional &safety